Abstract
Introduction: While BCL2 inhibition benefits many patients with acute myeloid leukemia (AML), resistance often occurs due to upregulation of other anti-apoptotic proteins such as MCL and BCLxL. Dual BCL2/xL inhibition with AZD4320 has potential for broader activity than BCL2-specific inhibition with venetoclax (Balachander et al, Clinical Cancer Research 2020). AZD0466 is a drug-dendrimer conjugate in which AZD4320 is covalently conjugated to a pegylated poly-L-lysine dendrimer and where, following IV infusion, AZD4320 is gradually released by hydrolysis. The dendrimer construct allows for efficient delivery of the highly potent but poorly soluble active drug, and this release profile was designed to mitigate potential maximum concentration (Cmax)-dependent BCLxL mediated effects (Patterson et al, Nature Communications Biology 2021). AZD0466 has shown antitumor activity in a range of preclinical models of hematological malignancy, including cell-line derived and patient-derived xenograft models of acute myeloid leukemia (AML), B-cell acute lymphoblastic leukemia (B-ALL), and T-cell acute lymphoblastic leukemia (T-ALL - Kannan et al AACR 2020 Abstract 3075). AZD0466 has previously been administered to 9 patients with advanced solid malignancies at doses declared tolerable through 200 mg in the first-time-in-human study (NCT04214093), and a physiologically based PK model validated across nonclinical species was used to predict human doses and exposures expected to drive tumor regression in hematologic malignancy. These studies provide rationale for testing AZD0466 in refractory AML and ALL patients in this phase I/II dose escalation and monotherapy expansion and drug-drug interaction study (NCT04865419).
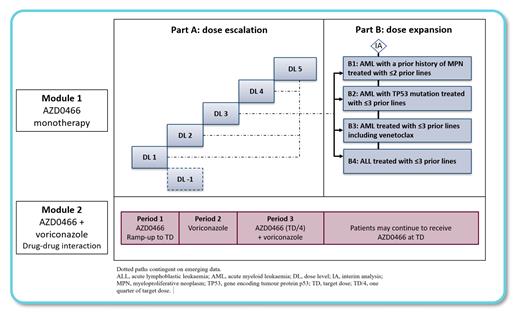
Study Design and Methods: NIMBLE (drug deNdrIMer targeting BCL2/xL in acute LEukemias) is a modular, non-randomized phase I/II dose escalation and expansion study. Module 1 will evaluate safety, tolerability, pharmacokinetics, and preliminary efficacy of AZD0466 as monotherapy in patients with relapsed/refractory AML or ALL. Patients are eligible if ≥ 18 years of age with any subtypes of relapsed/refractory AML or ALL without active CNS involvement and after at least one prior line of therapy. Treatment with hydroxyurea during screening and cycle 1 is permitted to control white blood cell count. Patients with extramedullary disease are also eligible. Primary endpoints are safety and tolerability of AZD0466 in patients with acute leukemia. In dose escalation, patients will be treated in each dose level according to an mTPI-2 design. Secondary endpoints are to determine pharmacokinetic parameters for total and released AZD4320. Exploratory endpoints include association of pharmacodynamic effects including changes to peripheral blasts and cleaved caspase activation, and initial evaluation of efficacy in relation to baseline leukemia characteristics. Planned dose expansion cohorts are AML patients with a prior history of MPN with ≤ 2 prior lines of therapy, AML patients with TP53 mutation treated with ≤ 3 prior lines of therapy, other AML treated with ≤ 3 prior lines (including venetoclax), and B- or T-ALL treated with ≤ 3 prior lines. Module 2 is a drug-drug interaction (DDI) study that will investigate the safety and establish the sensitivity of AZD0466 to voriconazole, a strong inhibitor of CYP3A4. There are no additional inclusion or exclusion criteria specific to Module 2. Further modules will be added via protocol amendment to investigate AZD0466 in combination with other antileukemia treatments, and may be triggered at the planned interim analyses during expansion.
AZD0466 will be administered with a dose ramp-up in cycle 1 from a starting dose on day 1, with subsequent titration to an intermediate dose on day 4, and target dose on day 8, with weekly 1-hour IV administration thereafter at the target dose. The duration of cycle 1 is 35 days, and subsequent cycles are 28 days in which AZD0466 will be administered once weekly. Patients will continue until treatment failure, unacceptable toxicity, or withdrawal of consent.
This study is actively enrolling patients at sites in the USA, and will be opening at multiple sites in Australia, Europe and South Korea. An update of preliminary safety and pharmacokinetics will be presented.
Konopleva: AstraZeneca: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Stemline Therapeutics: Research Funding; Forty Seven: Other: grant support, Research Funding; KisoJi: Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Ascentage: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Agios: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Cellectis: Other: grant support; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights. Jain: Fate Therapeutics: Research Funding; Cellectis: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Incyte: Research Funding; AbbVie: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Servier: Honoraria, Research Funding; Pfizer: Research Funding; Aprea Therapeutics: Research Funding; Pharmacyclics: Research Funding; Janssen: Honoraria; Beigene: Honoraria; TG Therapeutics: Honoraria. Andersen: AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Couto Francisco: AstraZeneca: Current Employment, Other: may own stock or stock options. Elgeioushi: AstraZeneca: Current Employment, Other: may own stock or stock options. Hobson: AstraZeneca: Current Employment, Other: may own stock or stock options. Scott: AstraZeneca: Current Employment, Other: may own stock or stock options. Stone: AstraZeneca: Current Employment, Other: may own stock or stock options. Sharma: AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Morentin Gutierrez: AstraZeneca: Current Employment, Other: may own stock or stock options. Tibes: AstraZeneca: Current Employment, Other: may own stock or stock options. Davies: AstraZeneca: Current Employment, Other: may own stock or stock options. Winkler: AstraZeneca: Current Employment, Other: may own stock or stock options. Fabbri: AstraZeneca: Current Employment, Other: may own stock or stock options. Zumla Cader: AstraZeneca: Current Employment, Other: may own stock or stock options. McNeer: AstraZeneca: Current Employment, Other: may own stock or stock options.